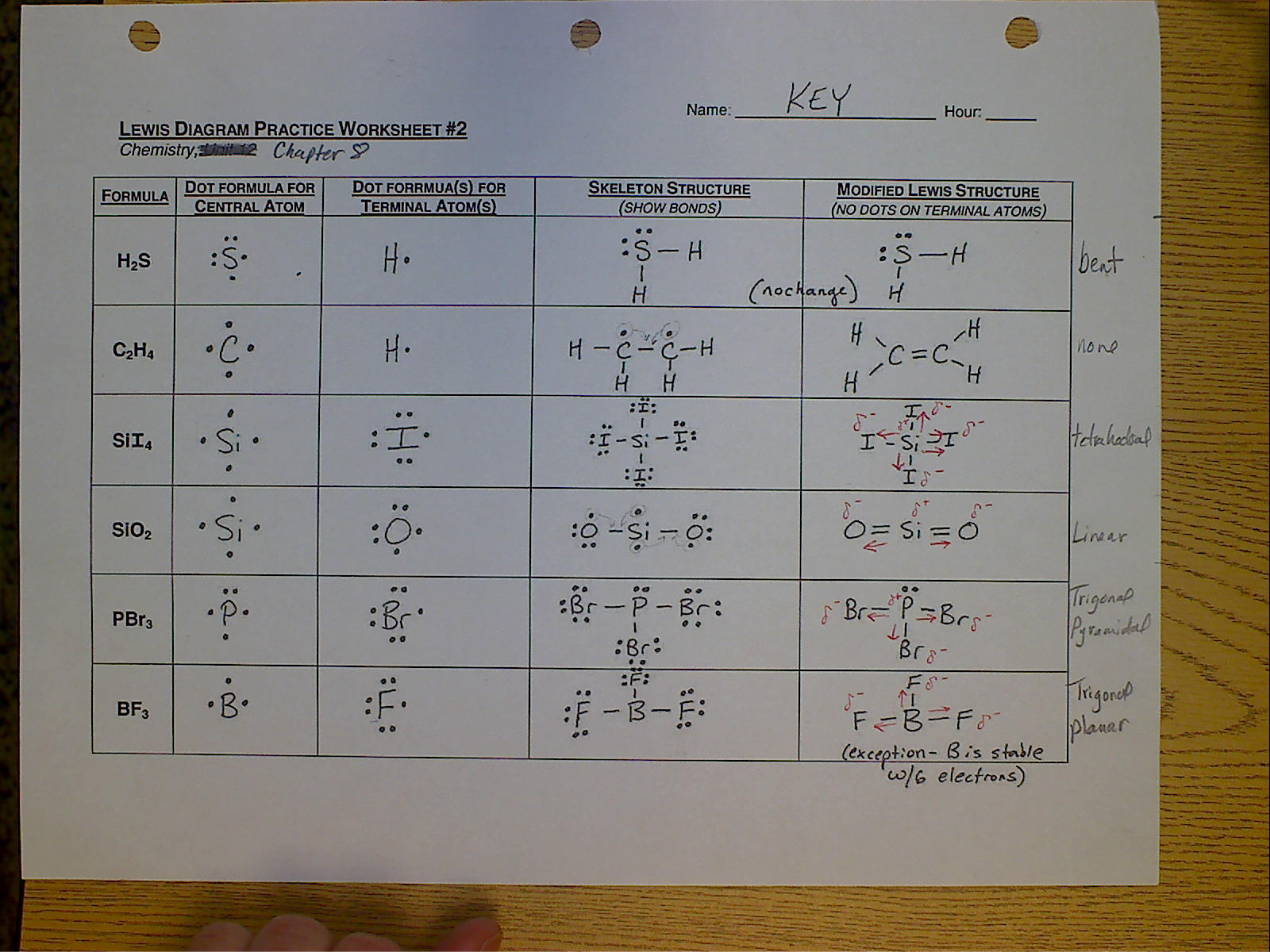
Lewis Structure Drawing Practice
Lewis Structure Drawing Practice - Draw the lewis structure for: Web write the lewis structures for carbon tetrachloride and phosgene. Web when you draw the lewis structure, make all the electrons paired unless there is an odd number of electrons. Web here are the steps to draw a lewis structure. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. The lewis structure rules and a periodic table while doing this exercise. The video covers the basic lewis structures for a general chemistry class. (there are molecules, like o 2, which have unpaired electrons even though they could all be paired, but you can't predict that with lewis structures, so assume they are all paired.) One line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines. Web the electrons in the outermost shell are called the _____ electrons. Web this online quiz is intended to give you extra practice in identifying and drawing lewis dot structures as well as predicting ion formation. Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Draw a lewis structure for each of the following:c. All electrons should be in lone pairs or bonding pairs. Draw the lewis structure, then id shape and polarity. Web when you draw the lewis structure, make all the electrons paired unless there is an odd number of electrons. A periodic table will be available for the exam, but the list of rules will not be available, so this is a chance to practice using the rules to help you. Yes, covalent bonds come in pairs which are represented by lines in lewis structures. Ch 2 br 2 d. For the following molecules or ions (where the central atom is underlined): See the following lewis dot structure diagrams for a few covalent compounds. Draw the lewis structure, then id shape and polarity. Do not add any more atoms. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.the diagram is also called a lewis dot diagram,. Web examples for drawing lewis structures for covalent bonds. Answer the following questions and check your answers below. Ch 2 br 2 d. Determine if the molecule is polar or nonpolar. Lewis diagram of the cyanide ion (cn⁻) Web practise drawing the lewis structure of molecules using the exercises below. Select answers to see the correct drawings. Change the following condensed structures to. Web practice drawing lewis structures with answers and explanation. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web in these practice problems, we will work on determining the lewis structures of molecules and ions. Draw a lewis structure for each of the following:b. You can usually return to this assignment anytime during the course, even after its due date. (there are molecules, like o 2, which have unpaired electrons even though they could all be paired, but. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.the diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Be sure you know how to draw correct lewis dot structures and are able to correctly predict the electronic arrangement. Web lewis structures practice worksheet draw the lewis structures for each of the following molecules. Web draw a lewis structure for each of the following:b. Change the following condensed structures to. Answer the following questions and check your answers below. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons. Add/replace, change length/angle, or erase bonds. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Draw a lewis structure for each of the following:c. This quiz aligns with the following ngss standard(s): Draw the lewis structure for: A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons.the diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper. Then name their electron arrangement, shape, and bond angles. Web here are the steps to draw a lewis structure. Web draw a lewis structure to answer a question. Web the electrons in the outermost shell are called the _____ electrons. Do not add any more atoms. Lewis diagram of formaldehyde (ch₂o) worked example: Write the correct skeletal structure for the molecule. Web the electrons in the outermost shell are called the _____ electrons. Draw the lewis dot structure for each of the following polyatomic ions: Reference the “how to draw a lewis dot structure” for a step by step guide. Do not add any more atoms. Web write the lewis structures for carbon tetrachloride and phosgene. Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Yes, covalent bonds come in pairs which are represented by lines in lewis structures. Add/replace, change length/angle, or erase bonds. The example is for the nitrate ion. Web in these practice problems, we will work on determining the lewis structures of molecules and ions. Problem \(\pageindex{7}\) the arrangement of atoms in several biologically important molecules is given here. Draw a lewis structure for each of the following:b. You can usually return to this assignment anytime during the course, even after its due date. Then name their electron arrangement, shape, and bond angles. Web practice drawing lewis structures with answers and explanation. Web draw a lewis structure for each of the following:b. One line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines. Determine if the molecule is polar or nonpolar. The video covers the basic lewis structures for a general chemistry class.Lewis Diagrams Made Easy How to Draw Lewis Dot Structures YouTube
How to draw Lewis Structures a step by step tutorial Middle School
Quick & Easy 5 Steps to Drawing Lewis Structures with Examples
Practice Drawing Lewis Structures Worksheets Worksheets For Kindergarten
How To Draw Lewis Structures YouTube
How To Draw Lewis Structures A Step By Step Tutorial
3 Ways to Draw Lewis Dot Structures wikiHow
Drawing Lewis Structures Worksheet
How To Draw Lewis Structures A Step By Step Tutorial
Draw Lewis Structure Practice
(There Are Molecules, Like O 2, Which Have Unpaired Electrons Even Though They Could All Be Paired, But You Can't Predict That With Lewis Structures, So Assume They Are All Paired.)
Ch 2 Br 2 D.
Web The Skeletal Structure Of Ethanethiol Is Shown Below.
Draw The Lewis Structure For:
Related Post: